Research
We do organic chemistry research and synthesis in the Ams Lab, and our focus is understanding how molecules are attracted to each other. This is a fundamental aspect of the field of molecular recognition, and of critical importance in areas such as catalysis and drug design. There is still much left to learn, and we take an experimental approach to the challenge by designing and synthesizing organic molecules that will help us understand the forces that govern intermolecular interactions and facilitate catalytic reactions.
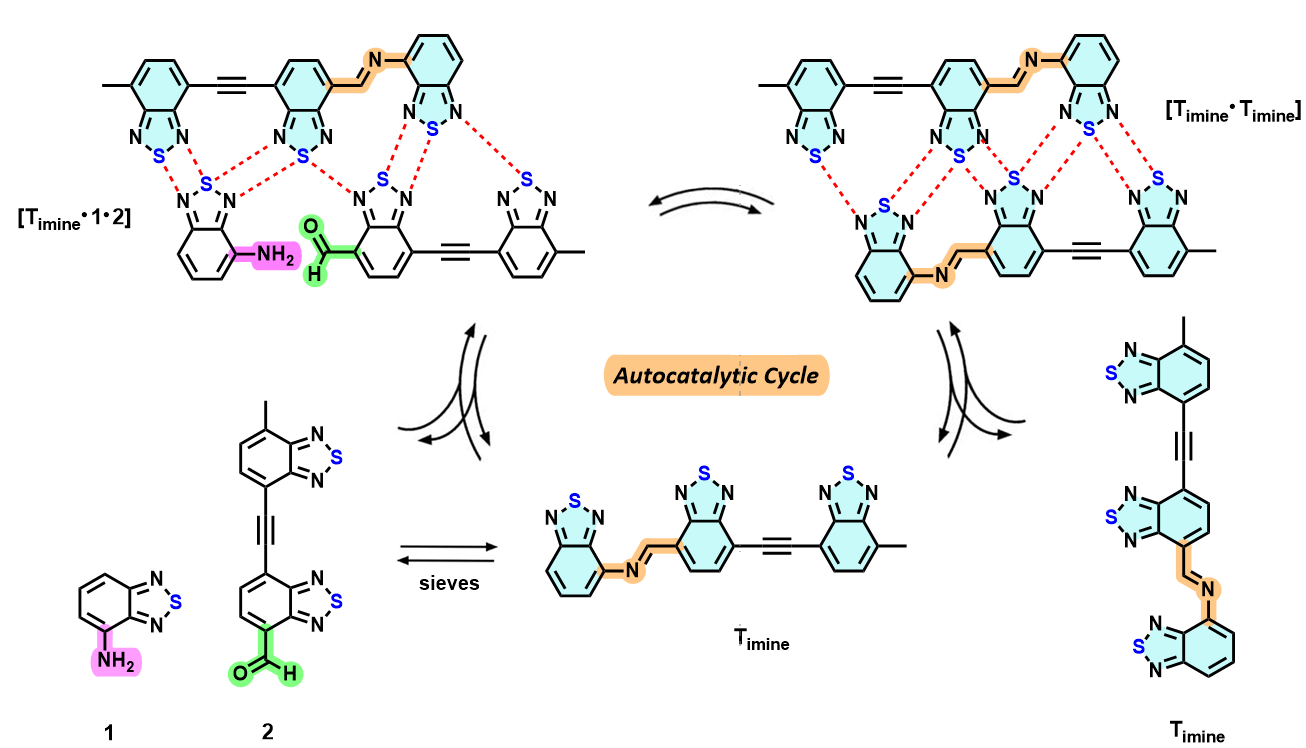
Project 1. Self-catalyzing molecules - Do they exist in the cosmos?
On the molecular level, life on Earth regenerates using deoxyribonucleic acid (DNA), a template molecule that self-replicates while carrying and transferring a genetic code. Its success resides in the unique shapes of its nitrogenous bases, which allow for selective pairing to form a duplex and a four-letter coding system. However, a fundamental unanswered human question remains: Is the DNA structure required for life, or does the universe contain perfectly capable chemicals that are entirely different from DNA and yet perform the same tasks?
In the Ams Lab, we are working on a new building block, based on the benzochalcogenadiazole (BZD) molecule, for the creation of exobiotic, self-replicating templates with genetic capacity. Organic structures similar in shape and content have recently been discovered in space. Our templates are designed to be autocatalytic – meaning that they accelerate their own formation in solution. Shown below is one of our imine-based systems (Figure 1), which we hypothesize has the mechanism shown.
Project 2. Non-Covalent Interactions (NCIs): Implications for organocatalysis & drug design.
Noncovalent interactions (NCIs) and their strengths are an under-explored research area, especially for halogens (XB), chalcogens (ChB), pnictogens (PnB), and tetrels (TB). These NCIs have become important to organocatalysis, crystal engineering, and drug discovery. A clear understanding of how these atoms interact, especially synergistically, is a goal of this project in the Ams Lab. We are using molecular probe molecules to quantify these NCIs so that this information can be used for the rational design of better organocatalysts, drugs, and crystal engineering.
Project 3. Expanding chemical space.
Next-generation molecular building blocks with multifunctional properties are in high demand for increasing the diversity and functionality of supramolecular architectures. We are working on a new class of building blocks based on chalcogen bonding interactions that may expand the current supramolecular toolkit by achieving two goals simultaneously: (1) expanding intermolecular connections while (2) providing secondary functions. These goals have implications for information storage, supramolecular chemistry, and optoelectronics. Our building block is based on 2,1,3-benzothiadiazole (BTD) derivatives, and we are developing hetero-square BTDs using solid- and solution-phase techniques (Figure 2).
Figure 2. A) Top view showing the in-plane position of the nitrogen lone pairs, sulfur sigma-holes and sigma-star orbitals. B) Homo- and C) hetero-square dimers.